Lets Get Fizzy
Posted:
July 26, 2017
Categories:
Chemistry

Make fizz and foam using a chemical reaction
Compare two baking soda and vinegar chemical reactions (one with detergent, one without), and watch for salt crystal formation.
Suitable for kids aged 4+NOTE
- These reactions can overflow to make a little bit of a wet mess! But that's part of the fun!

You Need:
- Vinegar
- Baking Soda
- Detergent
- Plastic cup x 2
- Measuring cup
- Teaspoon
What to do:
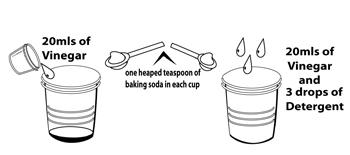
- Put 20 ml of vinegar in each plastic cup. Measure the vinegar using the measuring cup provided.
- Put 3 drops of detergent in only ONE of the plastic cups; remember which cup has the detergent or use some masking tape and texta to label the cup with the detergent.
- Put one heaped teaspoon of baking soda in each cup.
- Watch for any differences between the reactions in the two different cups.
- Leave the cups for about two hours and watch for salt crystal formation.
Why is it so?
The chemical reaction in the detergent cup should have lasted longer than the reaction in the cup without detergent. This is because the detergent acts to slow down the reaction. By mixing baking soda and vinegar together, a new chemical, carbon dioxide gas, is formed, hence all the fabulous fizz! If you are lucky, after about two hours you will see salt crystals form on the inside wall of the detergent reaction cup; it may be the foam itself that turns hard and crystallizes. When two special types of chemicals are mixed together, an acid and a base, salt crystals form. In this case the acid is vinegar and the base is baking soda.









 magento3
magento3